欢迎
目前有多数为CRO和Sponsor进行临床研究的信息系统。
CTDMS以不同目的为基础。本系统是为了满足研究中心的需要而创造的,而且
为了同行服务被有经验的研究者开发。
目前有多数为CRO和Sponsor进行临床研究的信息系统。
CTDMS以不同目的为基础。本系统是为了满足研究中心的需要而创造的,而且
为了同行服务被有经验的研究者开发。
CTDMS使规划简单。系统的设置尽可能简单。您只需要在CTDMS注册账号,创造新的研究,加入病人,然后可以在您需要的窗口规划访问。
您可以在CTDMS保存病人信息,并为了您的研究找需要的病人。
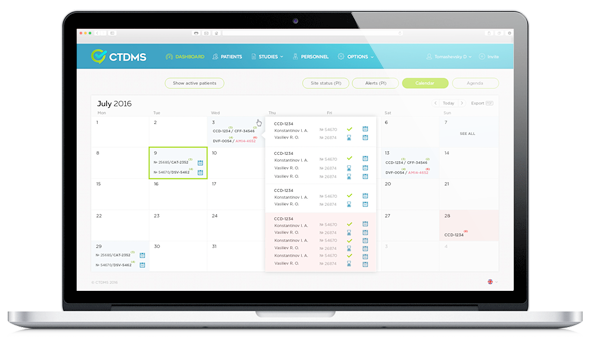
您可以监控职员的忙碌和研究中心的资源,按这些信息规划找新的病人参加您进行的研究。
您可以在系统保管文件、程式,是为了研究组所有的组员可以利用。

用CTDMS您可以实时知悉所有进行的研究过程。您还可以监控访问的完成,收到关于缺席访问和偏离时间表的通知。
Considering the needs of research sites regarding confidentiality issues we developed offline-versions of CTDMS service. You can download it from this page and install in your local network. When using offline-version of CTDMS all information remains in your LAN and never leaves it or interact with Internet (except getting updates).
System requirements for offline-version:
Server – any PC or server with MS Windows XP or later.
Client – any device with web-browser (Windows, MacOS, iOS, Androind, PC, tablets, smartphones). No additional software is required.
Notes:
1. CTDMS service is installed on PC, which plays server role and serves requests from all site`s users. You can choose any PC (or real server) with MS Windows to be such server. Please consider that choosen “server” PC should stay online for other site users to operate in service (so some of the sites will require it to be online 24/7).
2. In case CDTMS service is unavailable from user`s devices in LAN please check firewall and antivirus settings – incoming connections on port 8888/TCP should be allowed on “server” PC.